Global Medical Device Contract Manufacturing Market – Industry Trends and Forecast to 2032
Report ID: MS-891 | Healthcare and Pharma | Last updated: May, 2025 | Formats*:
The medical device contract manufacturing market involves the contracting of the design, development, and manufacturing of medical devices and their components by original equipment manufacturers (OEMs) to specialised third-party firms. The contract manufacturers provide a variety of services that include product design and engineering, prototyping, tooling, manufacturing (machining, moulding, and assembly), sterilising, packaging, and labelling. This represents the opportunity to leave core competencies, such as research and development, marketing, and distribution, to contract manufacturers while leveraging their knowledge and economies of scale. Contract manufacturing represents an opportunity for OEMs to gain advantages such as lower capital investment, access to specialised technologies and knowledge, higher flexibility in production, and improved time-to-market.
In addition, the global growth of the medical device business has created an increase in international contract manufacturing, enabling OEMs to take advantage of cost-competitive manufacturing sites and specialised skills globally. The trend will continue as medical device innovation is driving at a faster speed and the need for efficient and compliant manufacturing solutions intensifies.
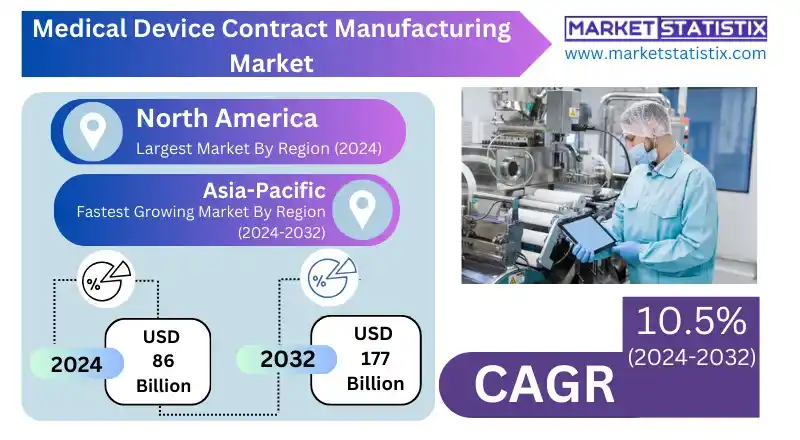
Medical Device Contract Manufacturing Report Highlights
| Report Metrics | Details |
|---|---|
| Forecast period | 2019-2032 |
| Base Year Of Estimation | 2024 |
| Growth Rate | CAGR of 10.5% |
| Forecast Value (2032) | USD 177 Billion |
| By Product Type | Device Development, Electronics Manufacturing, Raw Material Processing, Finished Goods Assembly, Component Manufacturing |
| Key Market Players |
|
| By Region |
|
Medical Device Contract Manufacturing Market Trends
The medical device contract manufacturing industry is presently undergoing a number of key trends. One of the notable trends is the growing usage of advanced manufacturing technologies, including 3D printing, robotics, and automation, by contract manufacturers. This facilitates quicker prototyping, manufacturing of intricate and customized devices, and enhanced efficiency. In addition, there is an increasing focus on end-to-end service solutions provided by contract manufacturers whereby they offer an integrated set of services ranging from product development and design through regulatory compliance, manufacturing, and distribution.
A second dominant trend is the increased focus on supply chain diversification and resilience. The recent disruptions have emphasised the necessity for stronger and geographically dispersed supply chains. Contract manufacturers are increasingly concentrating on creating agile and responsive supply networks. Sustainability is also emerging as a key consideration, with OEMs and contract manufacturers seeking environmentally friendly materials and green manufacturing strategies to help them reduce their own carbon footprint.
Medical Device Contract Manufacturing Market Leading Players
The key players profiled in the report are TE Connectivity, Cogmedix, Integer Holdings Corporation, Synecco Ltd, Phillips-Medisize, Celestica Inc., Sanmina Corporation, West Pharmaceutical Services Inc., Mack Molding, Nipro Corporation, Cirtec Medical, Jabil Inc., Plexus Corp., Viant Technology LLC, HDA Technology Inc., FLEX LTD., Keller Technology Corp., Thermo Fisher Scientific Inc.Growth Accelerators
The contract manufacturing of medical devices market is driven considerably by the growing interest of OEMs in cost-cutting and operational efficacy. Outsourcing manufacturing enables medical device firms to eliminate high costs of capital investment in manufacturing facilities, equipment, and labour. By taking advantage of the economies of scale and specialised knowledge of contract manufacturers, OEMs are able to obtain reduced manufacturing costs, enhanced resource utilisation, and increased overall profitability. This driver is highly applicable in a competitive industry where there are high pricing pressures.
The other important market driver is the increasing complexity of medical devices and the highly regulated environment. Today's medical devices tend to call for sophisticated manufacturing technologies, highly specialised materials, and highly complex assembly processes. Contract manufacturers often have the requisite knowledge, certifications, and quality management systems to manage these complexities and traverse stringent regulatory mandates from bodies such as the FDA and the EMA. Access to specialised capability and regulatory compliance knowledge facilitates OEMs, particularly smaller firms, to get innovative and compliant products to market more effectively and cost-efficiently
Medical Device Contract Manufacturing Market Segmentation analysis
The Global Medical Device Contract Manufacturing is segmented by Type, Application, and Region. By Type, the market is divided into Distributed Device Development, Electronics Manufacturing, Raw Material Processing, Finished Goods Assembly, Component Manufacturing . The Application segment categorizes the market based on its usage such as Drug Delivery Devices, Cardiovascular Devices, Orthopedic Devices, Diagnostic Devices, Surgical Instruments. Geographically, the market is assessed across key Regions like North America (United States, Canada, Mexico), South America (Brazil, Argentina, Chile, Rest of South America), Europe (Germany, France, Italy, United Kingdom, Benelux, Nordics, Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia, Southeast Asia, Rest of Asia-Pacific), MEA (Middle East, Africa) and others, each presenting distinct growth opportunities and challenges influenced by the regions.Competitive Landscape
The competitive market in the medical device contract manufacturing industry is varied and comprises a combination of large, multinational firms as well as smaller, niche players. Among the largest players with high market share are firms such as Flex, Jabil, Sanmina, Celestica, and Integer Holdings. Large contract manufacturers provide a broad range of services, a large global presence, and the capability to manage high-volume production for different types of medical devices.
Competition here is stiff and is based on such things as manufacturing capacity, technical expertise, quality and regulatory compliance, cost competitiveness, and the ability to provide end-to-end services from design through post-market service. Organizations are making more of an effort at strategic partnership, mergers, and acquisitions to diversify their service offerings, develop technological capabilities, and reach wider geographies. Having the capability to deal with challenging regulatory environments, uphold high standards of quality, and provide innovative manufacturing solutions is essential for businesses to keep their competitive advantage in this emerging market.
Challenges In Medical Device Contract Manufacturing Market
The medical device contract manufacturing industry is confronted with a number of compelling challenges that affect manufacturers and their customers alike. At the top of this list are tough and dynamic regulatory demands, such as FDA, ISO 13485, and EU MDR compliance, which require careful documentation, process verification, and continuous quality control. These regulatory challenges enhance the complexity and expense of manufacture, as any noncompliance can lead to product recall, legal issues, or financial losses.
Some other significant challenges are ensuring consistent quality between production lots, safeguarding intellectual property, and dealing with supply chain disruption, which has accelerated in recent years. It is also very competitive in the market, where new players and existing ones press prices down, and increasing material, equipment, and labour costs add more pressure on margins. Shortages of resources, low budgeting, and misplaced organisational priorities can stand in the way of process enhancement and quality management, heightening the chances of noncompliance and product recalls. These issues highlight the importance of sound regulatory intelligence, flexible manufacturing practices, and strategic alliances to ensure growth and innovation within the medical device contract manufacturing industry.
Risks & Prospects in Medical Device Contract Manufacturing Market
Opportunities are key in the form of growth in wearable and personal medical devices, material sciences developments, and combining technologies such as artificial intelligence and automation, which bring about efficiency in manufacture and allow for creating high-quality, customized products.
Geographically, North America has the biggest market share, underpinned by a strong healthcare infrastructure, sophisticated regulatory framework, and presence of leading medical device firms that are highly dependent on contract manufacturing for complex and specialised products. The fastest-growing geography is Asia-Pacific, driven by affordable healthcare manufacturing, state-sponsored programmes, and growing skilled manpower, especially in nations like Japan, China, and India. Europe is also experiencing strong growth, particularly in the UK and Germany, because of stringent regulatory requirements, growing demand for wearable devices, and emphasis on quality and efficiency control. These local dynamics, alongside continued healthcare investments and technological innovation, are defining a highly competitive and opportunity-rich medical device contract manufacturing landscape worldwide.
Key Target Audience
The major target market for medical device contract manufacturing involves original equipment manufacturers (OEMs) of medical devices who are looking for specialised partners to take care of design, prototyping, production, and assembly. These OEMs depend on contract manufacturers for technical expertise, cost savings, regulatory compliance, and the capability to rapidly scale up production. OEMs' foremost concern is minimising time-to-market, ensuring product quality, and fulfilling strict regulatory standards across worldwide markets.
,
, One secondary audience is startups and medium-sized medical technology companies that often do not have in-house manufacturing capacity. These companies seek flexible, end-to-end solutions from contract manufacturers, such as design for manufacturability, testing, and packaging. As the complexity of medical devices increases and demand for minimally invasive and wearable technologies rises, this segment increasingly relies on contract manufacturers to drive innovation faster and keep costs under control.
Merger and acquisition
The contract manufacturing market for medical devices has witnessed considerable consolidation via mergers and acquisitions (M&A) because of the compulsion to gain greater capabilities and global presence. Johnson & Johnson's acquisition of V-Wave for a maximum of $1.7 billion to strengthen its portfolio for heart failure treatment and the $12.5 billion acquisition of Shockwave Medical to enhance its cardiovascular device product offerings are some recent examples. Stryker Corporation also diversified its orthopaedic implant operations by acquiring France-based SERF SAS during March 2024.
Private equity players are active in this space, with CVC Capital Partners looking to sell its 60% holding in Italy's Genetic Group for about €700 million. Sanner Group's buyout of Gilero LLC in September 2024 also strengthened its drug delivery and diagnostics foothold. Regulatory pressures are rising, however, as seen in the FTC lawsuit to prevent GTCR's $627 million buyout of Surmodics on grounds of decreased competition in coatings of medical devices
Analyst Comment
The global market for contract manufacturing of medical devices is growing very strongly, and its size is approximately USD 76.8 billion in 2024 and is expected to be up to USD 253.86 billion by 2034. This growth is fuelled by the rise in the burden of chronic diseases, the growing need for innovative and personalised medical devices, and the demand for cost-effective, scalable manufacturing. Medical device companies are progressively outsourcing manufacturing to contract manufacturing organizations (CMOs) to streamline operations, reduce product launch times, and concentrate on core competencies like R&D and marketing. The use of advanced technologies such as automation, robotics, additive manufacturing, and artificial intelligence is further increasing production efficiency and allowing for the creation of complex, customized devices.
- 1.1 Report description
- 1.2 Key market segments
- 1.3 Key benefits to the stakeholders
2: Executive Summary
- 2.1 Medical Device Contract Manufacturing- Snapshot
- 2.2 Medical Device Contract Manufacturing- Segment Snapshot
- 2.3 Medical Device Contract Manufacturing- Competitive Landscape Snapshot
3: Market Overview
- 3.1 Market definition and scope
- 3.2 Key findings
- 3.2.1 Top impacting factors
- 3.2.2 Top investment pockets
- 3.3 Porter’s five forces analysis
- 3.3.1 Low bargaining power of suppliers
- 3.3.2 Low threat of new entrants
- 3.3.3 Low threat of substitutes
- 3.3.4 Low intensity of rivalry
- 3.3.5 Low bargaining power of buyers
- 3.4 Market dynamics
- 3.4.1 Drivers
- 3.4.2 Restraints
- 3.4.3 Opportunities
4: Medical Device Contract Manufacturing Market by Type
- 4.1 Overview
- 4.1.1 Market size and forecast
- 4.2 Device Development
- 4.2.1 Key market trends, factors driving growth, and opportunities
- 4.2.2 Market size and forecast, by region
- 4.2.3 Market share analysis by country
- 4.3 Electronics Manufacturing
- 4.3.1 Key market trends, factors driving growth, and opportunities
- 4.3.2 Market size and forecast, by region
- 4.3.3 Market share analysis by country
- 4.4 Finished Goods Assembly
- 4.4.1 Key market trends, factors driving growth, and opportunities
- 4.4.2 Market size and forecast, by region
- 4.4.3 Market share analysis by country
- 4.5 Raw Material Processing
- 4.5.1 Key market trends, factors driving growth, and opportunities
- 4.5.2 Market size and forecast, by region
- 4.5.3 Market share analysis by country
- 4.6 Component Manufacturing
- 4.6.1 Key market trends, factors driving growth, and opportunities
- 4.6.2 Market size and forecast, by region
- 4.6.3 Market share analysis by country
5: Medical Device Contract Manufacturing Market by Application / by End Use
- 5.1 Overview
- 5.1.1 Market size and forecast
- 5.2 Orthopedic Devices
- 5.2.1 Key market trends, factors driving growth, and opportunities
- 5.2.2 Market size and forecast, by region
- 5.2.3 Market share analysis by country
- 5.3 Cardiovascular Devices
- 5.3.1 Key market trends, factors driving growth, and opportunities
- 5.3.2 Market size and forecast, by region
- 5.3.3 Market share analysis by country
- 5.4 Diagnostic Devices
- 5.4.1 Key market trends, factors driving growth, and opportunities
- 5.4.2 Market size and forecast, by region
- 5.4.3 Market share analysis by country
- 5.5 Drug Delivery Devices
- 5.5.1 Key market trends, factors driving growth, and opportunities
- 5.5.2 Market size and forecast, by region
- 5.5.3 Market share analysis by country
- 5.6 Surgical Instruments
- 5.6.1 Key market trends, factors driving growth, and opportunities
- 5.6.2 Market size and forecast, by region
- 5.6.3 Market share analysis by country
6: Medical Device Contract Manufacturing Market by Region
- 6.1 Overview
- 6.1.1 Market size and forecast By Region
- 6.2 North America
- 6.2.1 Key trends and opportunities
- 6.2.2 Market size and forecast, by Type
- 6.2.3 Market size and forecast, by Application
- 6.2.4 Market size and forecast, by country
- 6.2.4.1 United States
- 6.2.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.2.4.1.2 Market size and forecast, by Type
- 6.2.4.1.3 Market size and forecast, by Application
- 6.2.4.2 Canada
- 6.2.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.2.4.2.2 Market size and forecast, by Type
- 6.2.4.2.3 Market size and forecast, by Application
- 6.2.4.3 Mexico
- 6.2.4.3.1 Key market trends, factors driving growth, and opportunities
- 6.2.4.3.2 Market size and forecast, by Type
- 6.2.4.3.3 Market size and forecast, by Application
- 6.2.4.1 United States
- 6.3 South America
- 6.3.1 Key trends and opportunities
- 6.3.2 Market size and forecast, by Type
- 6.3.3 Market size and forecast, by Application
- 6.3.4 Market size and forecast, by country
- 6.3.4.1 Brazil
- 6.3.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.3.4.1.2 Market size and forecast, by Type
- 6.3.4.1.3 Market size and forecast, by Application
- 6.3.4.2 Argentina
- 6.3.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.3.4.2.2 Market size and forecast, by Type
- 6.3.4.2.3 Market size and forecast, by Application
- 6.3.4.3 Chile
- 6.3.4.3.1 Key market trends, factors driving growth, and opportunities
- 6.3.4.3.2 Market size and forecast, by Type
- 6.3.4.3.3 Market size and forecast, by Application
- 6.3.4.4 Rest of South America
- 6.3.4.4.1 Key market trends, factors driving growth, and opportunities
- 6.3.4.4.2 Market size and forecast, by Type
- 6.3.4.4.3 Market size and forecast, by Application
- 6.3.4.1 Brazil
- 6.4 Europe
- 6.4.1 Key trends and opportunities
- 6.4.2 Market size and forecast, by Type
- 6.4.3 Market size and forecast, by Application
- 6.4.4 Market size and forecast, by country
- 6.4.4.1 Germany
- 6.4.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.1.2 Market size and forecast, by Type
- 6.4.4.1.3 Market size and forecast, by Application
- 6.4.4.2 France
- 6.4.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.2.2 Market size and forecast, by Type
- 6.4.4.2.3 Market size and forecast, by Application
- 6.4.4.3 Italy
- 6.4.4.3.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.3.2 Market size and forecast, by Type
- 6.4.4.3.3 Market size and forecast, by Application
- 6.4.4.4 United Kingdom
- 6.4.4.4.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.4.2 Market size and forecast, by Type
- 6.4.4.4.3 Market size and forecast, by Application
- 6.4.4.5 Benelux
- 6.4.4.5.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.5.2 Market size and forecast, by Type
- 6.4.4.5.3 Market size and forecast, by Application
- 6.4.4.6 Nordics
- 6.4.4.6.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.6.2 Market size and forecast, by Type
- 6.4.4.6.3 Market size and forecast, by Application
- 6.4.4.7 Rest of Europe
- 6.4.4.7.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.7.2 Market size and forecast, by Type
- 6.4.4.7.3 Market size and forecast, by Application
- 6.4.4.1 Germany
- 6.5 Asia Pacific
- 6.5.1 Key trends and opportunities
- 6.5.2 Market size and forecast, by Type
- 6.5.3 Market size and forecast, by Application
- 6.5.4 Market size and forecast, by country
- 6.5.4.1 China
- 6.5.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.1.2 Market size and forecast, by Type
- 6.5.4.1.3 Market size and forecast, by Application
- 6.5.4.2 Japan
- 6.5.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.2.2 Market size and forecast, by Type
- 6.5.4.2.3 Market size and forecast, by Application
- 6.5.4.3 India
- 6.5.4.3.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.3.2 Market size and forecast, by Type
- 6.5.4.3.3 Market size and forecast, by Application
- 6.5.4.4 South Korea
- 6.5.4.4.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.4.2 Market size and forecast, by Type
- 6.5.4.4.3 Market size and forecast, by Application
- 6.5.4.5 Australia
- 6.5.4.5.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.5.2 Market size and forecast, by Type
- 6.5.4.5.3 Market size and forecast, by Application
- 6.5.4.6 Southeast Asia
- 6.5.4.6.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.6.2 Market size and forecast, by Type
- 6.5.4.6.3 Market size and forecast, by Application
- 6.5.4.7 Rest of Asia-Pacific
- 6.5.4.7.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.7.2 Market size and forecast, by Type
- 6.5.4.7.3 Market size and forecast, by Application
- 6.5.4.1 China
- 6.6 MEA
- 6.6.1 Key trends and opportunities
- 6.6.2 Market size and forecast, by Type
- 6.6.3 Market size and forecast, by Application
- 6.6.4 Market size and forecast, by country
- 6.6.4.1 Middle East
- 6.6.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.6.4.1.2 Market size and forecast, by Type
- 6.6.4.1.3 Market size and forecast, by Application
- 6.6.4.2 Africa
- 6.6.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.6.4.2.2 Market size and forecast, by Type
- 6.6.4.2.3 Market size and forecast, by Application
- 6.6.4.1 Middle East
- 7.1 Overview
- 7.2 Key Winning Strategies
- 7.3 Top 10 Players: Product Mapping
- 7.4 Competitive Analysis Dashboard
- 7.5 Market Competition Heatmap
- 7.6 Leading Player Positions, 2022
8: Company Profiles
- 8.1 Plexus Corp.
- 8.1.1 Company Overview
- 8.1.2 Key Executives
- 8.1.3 Company snapshot
- 8.1.4 Active Business Divisions
- 8.1.5 Product portfolio
- 8.1.6 Business performance
- 8.1.7 Major Strategic Initiatives and Developments
- 8.2 Keller Technology Corp.
- 8.2.1 Company Overview
- 8.2.2 Key Executives
- 8.2.3 Company snapshot
- 8.2.4 Active Business Divisions
- 8.2.5 Product portfolio
- 8.2.6 Business performance
- 8.2.7 Major Strategic Initiatives and Developments
- 8.3 Mack Molding
- 8.3.1 Company Overview
- 8.3.2 Key Executives
- 8.3.3 Company snapshot
- 8.3.4 Active Business Divisions
- 8.3.5 Product portfolio
- 8.3.6 Business performance
- 8.3.7 Major Strategic Initiatives and Developments
- 8.4 Cirtec Medical
- 8.4.1 Company Overview
- 8.4.2 Key Executives
- 8.4.3 Company snapshot
- 8.4.4 Active Business Divisions
- 8.4.5 Product portfolio
- 8.4.6 Business performance
- 8.4.7 Major Strategic Initiatives and Developments
- 8.5 Integer Holdings Corporation
- 8.5.1 Company Overview
- 8.5.2 Key Executives
- 8.5.3 Company snapshot
- 8.5.4 Active Business Divisions
- 8.5.5 Product portfolio
- 8.5.6 Business performance
- 8.5.7 Major Strategic Initiatives and Developments
- 8.6 West Pharmaceutical Services Inc.
- 8.6.1 Company Overview
- 8.6.2 Key Executives
- 8.6.3 Company snapshot
- 8.6.4 Active Business Divisions
- 8.6.5 Product portfolio
- 8.6.6 Business performance
- 8.6.7 Major Strategic Initiatives and Developments
- 8.7 Celestica Inc.
- 8.7.1 Company Overview
- 8.7.2 Key Executives
- 8.7.3 Company snapshot
- 8.7.4 Active Business Divisions
- 8.7.5 Product portfolio
- 8.7.6 Business performance
- 8.7.7 Major Strategic Initiatives and Developments
- 8.8 FLEX LTD.
- 8.8.1 Company Overview
- 8.8.2 Key Executives
- 8.8.3 Company snapshot
- 8.8.4 Active Business Divisions
- 8.8.5 Product portfolio
- 8.8.6 Business performance
- 8.8.7 Major Strategic Initiatives and Developments
- 8.9 Jabil Inc.
- 8.9.1 Company Overview
- 8.9.2 Key Executives
- 8.9.3 Company snapshot
- 8.9.4 Active Business Divisions
- 8.9.5 Product portfolio
- 8.9.6 Business performance
- 8.9.7 Major Strategic Initiatives and Developments
- 8.10 Nipro Corporation
- 8.10.1 Company Overview
- 8.10.2 Key Executives
- 8.10.3 Company snapshot
- 8.10.4 Active Business Divisions
- 8.10.5 Product portfolio
- 8.10.6 Business performance
- 8.10.7 Major Strategic Initiatives and Developments
- 8.11 TE Connectivity
- 8.11.1 Company Overview
- 8.11.2 Key Executives
- 8.11.3 Company snapshot
- 8.11.4 Active Business Divisions
- 8.11.5 Product portfolio
- 8.11.6 Business performance
- 8.11.7 Major Strategic Initiatives and Developments
- 8.12 HDA Technology Inc.
- 8.12.1 Company Overview
- 8.12.2 Key Executives
- 8.12.3 Company snapshot
- 8.12.4 Active Business Divisions
- 8.12.5 Product portfolio
- 8.12.6 Business performance
- 8.12.7 Major Strategic Initiatives and Developments
- 8.13 Synecco Ltd
- 8.13.1 Company Overview
- 8.13.2 Key Executives
- 8.13.3 Company snapshot
- 8.13.4 Active Business Divisions
- 8.13.5 Product portfolio
- 8.13.6 Business performance
- 8.13.7 Major Strategic Initiatives and Developments
- 8.14 Viant Technology LLC
- 8.14.1 Company Overview
- 8.14.2 Key Executives
- 8.14.3 Company snapshot
- 8.14.4 Active Business Divisions
- 8.14.5 Product portfolio
- 8.14.6 Business performance
- 8.14.7 Major Strategic Initiatives and Developments
- 8.15 Cogmedix
- 8.15.1 Company Overview
- 8.15.2 Key Executives
- 8.15.3 Company snapshot
- 8.15.4 Active Business Divisions
- 8.15.5 Product portfolio
- 8.15.6 Business performance
- 8.15.7 Major Strategic Initiatives and Developments
- 8.16 Phillips-Medisize
- 8.16.1 Company Overview
- 8.16.2 Key Executives
- 8.16.3 Company snapshot
- 8.16.4 Active Business Divisions
- 8.16.5 Product portfolio
- 8.16.6 Business performance
- 8.16.7 Major Strategic Initiatives and Developments
- 8.17 Sanmina Corporation
- 8.17.1 Company Overview
- 8.17.2 Key Executives
- 8.17.3 Company snapshot
- 8.17.4 Active Business Divisions
- 8.17.5 Product portfolio
- 8.17.6 Business performance
- 8.17.7 Major Strategic Initiatives and Developments
- 8.18 Thermo Fisher Scientific Inc.
- 8.18.1 Company Overview
- 8.18.2 Key Executives
- 8.18.3 Company snapshot
- 8.18.4 Active Business Divisions
- 8.18.5 Product portfolio
- 8.18.6 Business performance
- 8.18.7 Major Strategic Initiatives and Developments
9: Analyst Perspective and Conclusion
- 9.1 Concluding Recommendations and Analysis
- 9.2 Strategies for Market Potential
Scope of Report
| Aspects | Details |
|---|---|
By Type |
|
By Application |
|
Report Licenses
Our Team

Frequently Asked Questions (FAQ):
What is the projected market size of Medical Device Contract Manufacturing in 2032?
+
-
Which application type is expected to remain the largest segment in the Global Medical Device Contract Manufacturing market?
+
-
How big is the Global Medical Device Contract Manufacturing market?
+
-
How do regulatory policies impact the Medical Device Contract Manufacturing Market?
+
-
What major players in Medical Device Contract Manufacturing Market?
+
-
What applications are categorized in the Medical Device Contract Manufacturing market study?
+
-
Which product types are examined in the Medical Device Contract Manufacturing Market Study?
+
-
Which regions are expected to show the fastest growth in the Medical Device Contract Manufacturing market?
+
-
Which application holds the second-highest market share in the Medical Device Contract Manufacturing market?
+
-
Which region is the fastest growing in the Medical Device Contract Manufacturing market?
+
-

